Why 3Aware?
Gathering real-world data impacts more than manufacturers.
Post-market clinical follow-up applies to all medical devices, of all classes, and is required under EU MDR with very few exceptions. Beyond EUMDR, in 2022, 45% of the original Premarket Approvals (PMAs) granted by the FDA require post-approval studies. With limited time and resources, manufacturers are forced to make the choice of which devices to prioritize.
Hospitals, providers, and their patients are affected by the entire healthcare ecosystem. Physicians are being asked to participate in clinical data collection studies on existing, well-established devices simply because data is not readily available. The result of this is providers spending less time on innovation and patient care.
When these PMCF needs cannot be met, devices are no longer available to the doctors and patients who depend on them. This disparately impacts vulnerable populations such as pediatric patients, and those with rare conditions.
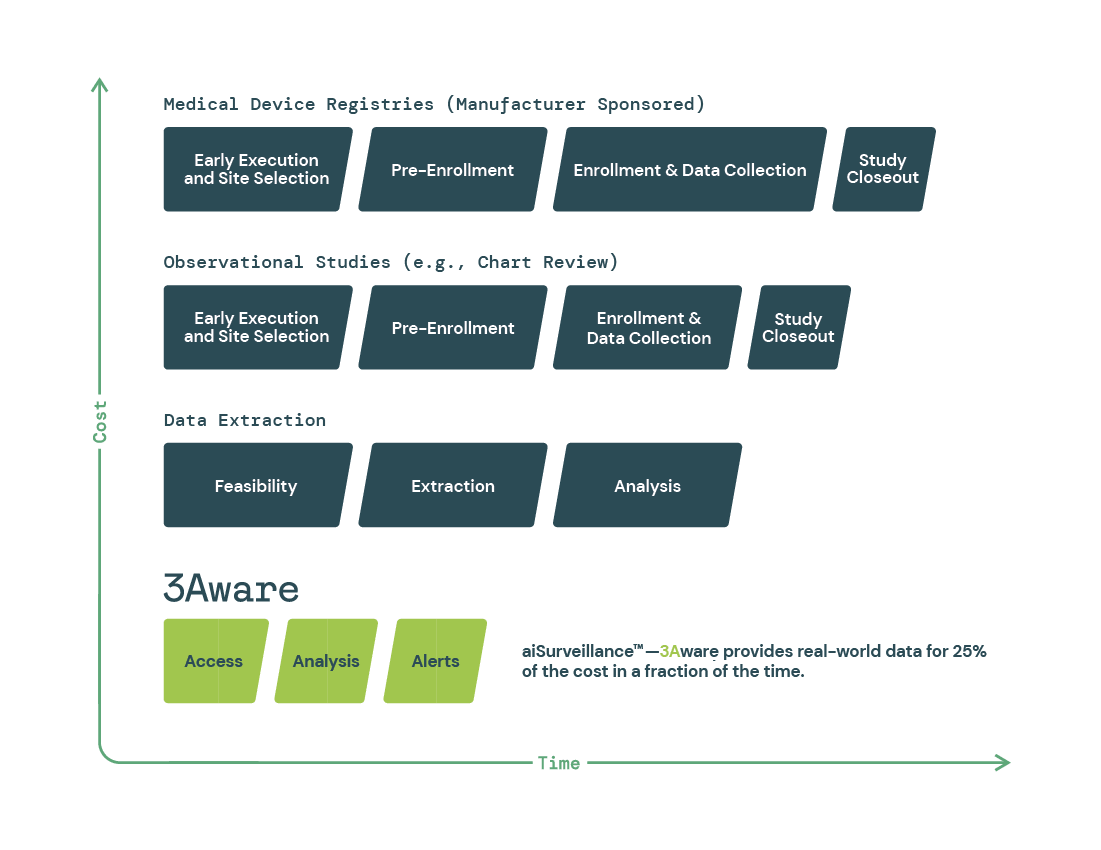
3Aware provides aiSurveillance™ for medical device manufacturers to save time and money by offering Access, Analysis and Alerts of real-world clinical data. This approach is faster and more cost effective for manufacturers, requires less effort by hospitals and their providers which leads to better patient outcomes.
3Aware is the logical choice.
Pre-market investigations alone do not provide the entire safety and performance picture of a medical product, which is why PMS is critical. 3Aware allows stakeholders to meet their PMCF needs by monitoring device usage and patient outcomes in the real-world for less time and cost than other currently available methods.