3Aware Expands Imaging Capabilities to Strengthen Real-World Evidence Generation
July 23, 2025 - 3Aware has introduced a powerful new enhancement to its real-world evidence platform: integrated access to both unstructured radiology reports and an embedded DICOM image viewer. These new features empower medical device researchers with deeper, more actionable insights by extending data visibility beyond clinical notes and structured EHR fields – bringing imaging to the forefront of regulatory-grade evidence generation.
3Aware Platform Now Supports Integrated Imaging:
-
Unstructured Radiology Reports
Teams can now include radiology reports – such as MRI, CT, and X-ray impressions – across the entire patient data window, not just during the index procedure. These reports contain critical diagnostic details and narrative findings that complement structured data and clinical notes. With access to over 25 imaging modalities, sponsors gain an interpretive layer of clinical relevance – without requiring radiology expertise or manual chart review.
-
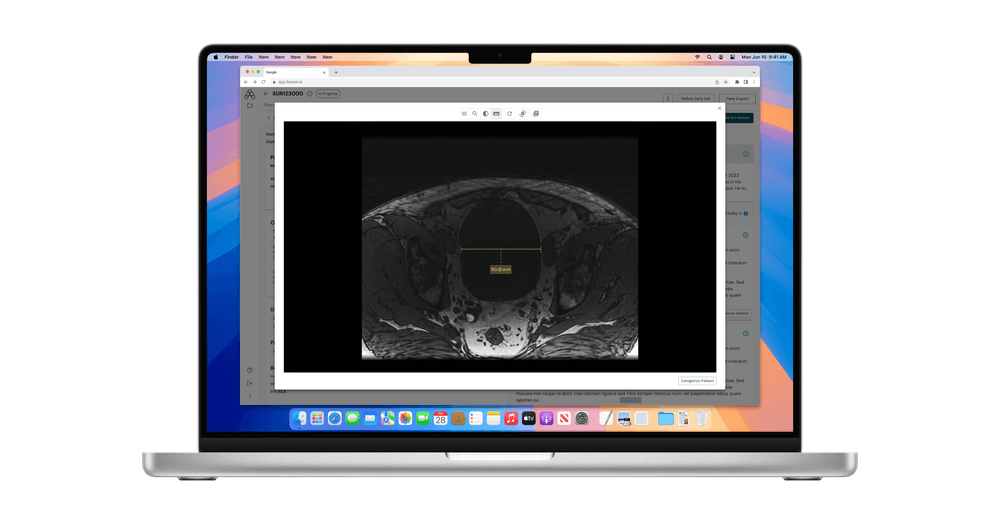
Integrated DICOM Image Viewer
The new image viewer supports 15 image modalities, from ultrasound to PET and X-ray angiography. Users can view images linked to radiology reports, apply annotations and measurements, and access full DICOM header data, all within 3Aware’s secure workbench. As always, all data is fully de-identified in accordance with HIPAA and applicable global standards.
Full Longitudinal Data Suite
This imaging expansion builds on 3Aware’s already robust access to longitudinal patient data. Our platform enables detailed cohort discovery and outcome measurement across:
- Structured and Unstructured Clinical Notes
- Lab Results and Vital Signs
- Diagnosis and Procedure Codes
- Prescriptions and Therapy Records
- Radiology Narratives and Imaging
- PROMs, AE tracking, and follow-up documentation
This depth of data allows study sponsors to retrace the entire patient care journey – before, during, and after device intervention – enabling more complete Post-Market Clinical Follow-up (PMCF), more accurate risk-benefit assessments, and stronger alignment with FDA and EU MDR evidence expectations.
Ready to Enhance Your RWE Strategy?
Whether you need to validate imaging endpoints, confirm long-term follow-up, or explore device safety and effectiveness in real-world settings, 3Aware’s imaging features provide a new standard of depth and precision.
Book a demo with us to learn how these tools can support your next study!