Empowering MedTech with Streamlined, Fit-For-Purpose Clinical Research Analytics
Optimize your total product lifecycle with less cost, time, and risk
3Aware Capabilities
aiSurveillance
3Aware developed aiSurveillance technology for the MedTech sector. A blend of clinical science and data science, it leverages advanced algorithms–applied to electronic health records (EHRs), supply chain management, charge master and other health system inventory & accounting systems–to process and analyze vast amounts of real-world patient data swiftly and accurately. AI- optimized repeatable analytic protocols and decision-making mine and analyze the complete EHR (including information-rich unstructured clinician notes) with ever increasing efficiency. aiSurveillance automates the translation of patient-level, device-specific real-world data into easily digestible patient outcomes… resulting in highest quality, regulatory-grade real-world evidence.
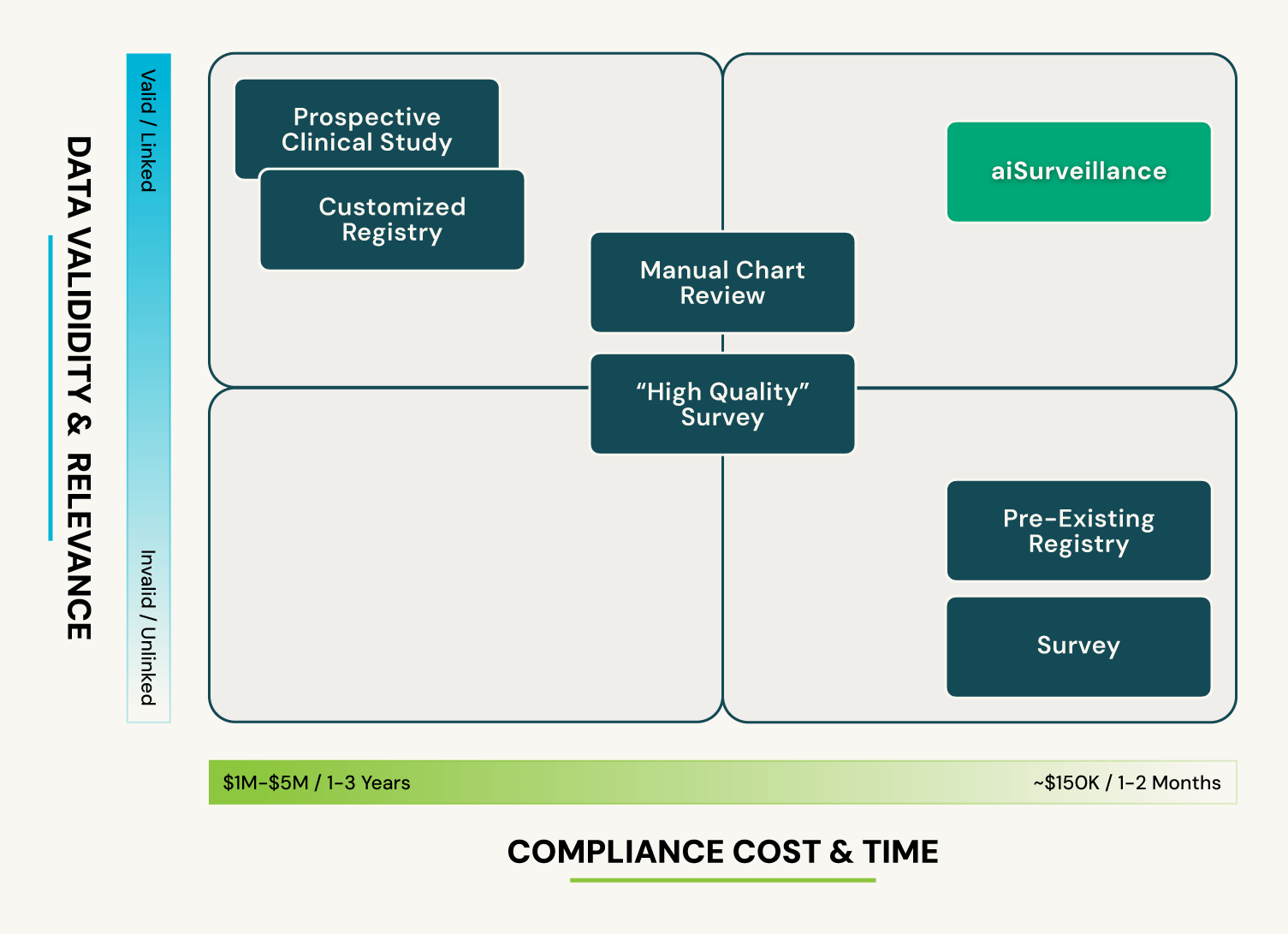
3Aware vs. Traditional Methods
Compared to traditional RWE methods, 3Aware reduces time, risk and resources dependency, thus accelerating study timelines… and ongoing access to pre-identified, device-specific cohorts is available for ad hoc studies. Both data bias and operational risk are significantly decreased because the study cohort is immediately known and described via a feasibility assessment, before committing to the full study.
3Aware Clarity
Device & Patient Insights
3Aware pinpoints specific devices and device categories to specific patient events via UDI numbers, integrated from a variety of health system data sources. Comprehensive longitudinal data, including Patient Reported Outcome Measures (PROMs), EHR imaging and procedure-specific clinical evidence, is available for a multitude of compliance and portfolio optimization uses… for example, tracking Class III implants.
Scientific Workbench
The AI-powered scientific work bench employs Reinforced Learning from Human Feedback, Natural Language Processing & Understanding (NLP/U) and soon: End-Point Specific LLMs, to streamline the analysis of complex data, maximizing real-time clinical insights and regulatory readiness. Patient level findings are provided, as well as clear summaries, to facilitate report generation.
Governance
3Aware ensures rigorous standards to protect data integrity and patient health information. Our platform is built on a HITRUST® Risk-based, 2-year certified platform that employs robust cybersecurity measures, including continuous vulnerability scanning and annual third-party audits, which have consistently shown no vulnerabilities. All data is de-identified according to stringent expert determination standards and encrypted in transmission and at rest, ensuring data privacy. Additionally, access to client information is strictly controlled, with a least privilege and need-to-know policy enforced alongside a thorough quality assurance process to maintain data integrity and reliability.
"Of the data options available, 3Aware is the first and only platform I know of that connects specific devices to patient medical records and offers users direct access to de-identified patient-level data, including unstructured clinical notes."
3Aware Coverage
3Aware has coverage of over 60K devices, sourced from a patient population of over 20M, and growing. Data is integrated from electronic health records and a myriad of administrative, operational, accounting/billing, supply chain management and operating room scheduling systems. A specific device is connected to a specific patient by connecting data points via their various unique identifiers.
3Aware Engagement Model
Catalog Number
Minimize Selection
Data Elements & Required Follow-Up
De-Risk Full Project
Scientific Support
Repeatable Projects
Continuing Surveillance